
The oxidation number of the sulfur atom in the SO 4 2- ion must be +6, for example, because the sum of the oxidation numbers of the atoms in this ion must equal -2.ġ1. The sum of the oxidation numbers in a polyatomic ion is equal to the charge on the ion. The sum of the oxidation numbers in a neutral compound is zero.ġ0. The elements in Group VIIA often form compounds (such as AlF 3, HCl, and ZnBr 2) in which the nonmetal has a -1 oxidation number.ĩ. Exceptions include molecules and polyatomic ions that contain O-O bonds, such as O 2, O 3, H 2O 2, and the O 2 2- ion.Ĩ. Oxygen usually has an oxidation number of -2. The elements in Group IIA form compounds (such as Mg 3N 2 and CaCO 3) in which the metal atom has a +2 oxidation number.ħ. The metals in Group IA form compounds (such as Li 3N and Na 2S) in which the metal atom has an oxidation number of +1.Ħ.

The oxidation number of hydrogen is -1 when it is combined with a metal as in. The oxidation number of hydrogen is +1 when it is combined with a nonmetal as in CH 4, NH 3, H 2O, and HCl.Ĥ. The oxidation number of sodium in the Na + ion is +1, for example, and the oxidation number of chlorine in the Cl - ion is -1.ģ. The oxidation number of simple ions is equal to the charge on the ion. Thus, the atoms in O 2, O 3, P 4, S 8, and aluminum metal all have an oxidation number of 0.Ģ. The oxidation number of an atom is zero in a neutral substance that contains atoms of only one element.
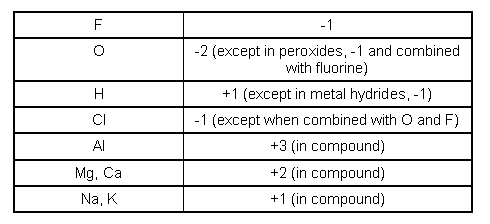
The rules are presented in order with the previous rule superseding the next.ġ. The determination of the oxidation number (or oxidation state) of chemical compounds can be made by following a few simple rules.


 0 kommentar(er)
0 kommentar(er)
